Contact us
PAVIA FARMACEUTICI s.r.l.
via Vistarino 14/F
27010 Copiano (PV) - Italy
Tel: (+39) 348 7760027
Fax: (+39) 0382 974333
Email: info@paviafarmaceutici.it
Working hours:
- Mon-Fri: 8.30 - 12.30 | 2.00 pm - 6.00 pm

Pavia Farmaceutici is a company active in the research and development of medical devices and new disinfectants as well as in the development of delivery systems for cosmetics and nutraceuticals.
In close cooperation with Italian and foreign Universities and research institutes, Pavia Farmaceutici has carried out researches and developed patents with the dual aim of improving and above all developing technologies for everybody’s care and well-being.
Technological innovation is based on patented ionic molecules and adducts (PCT / IB2013 / 054647, PCT / IB2013 / 054649, US2016 / 0130234 A1) where effective synergies operate between different actives, which allow, even at low levels of concentration, to obtain an effective antimicrobial action, useful for applications in new pharmaceutical formulations for topical use.
Antiseptics differ from antibiotics because they show a wider spectrum of activity and can be effective against many kinds of microorganisms, such as aerobic and anaerobic bacteria, yeasts, fungi and moulds.
Bacterial resistance to antibiotics is widely documented in medical literature. For instance, Tambe et al. compared the ability of the Staphylococcus epidermidis to develop resistance to various antibiotics and antiseptics. (J Antimicrob Chemother. 2001;47(5): 589-598).
The results obtained show that such bacterial species develops resistance to antibiotics, namely minocycline and rifampicin, while no evidence of resistance was found in Biguanides and Silver molecular complexes.
Silver is an effective antimicrobial agent with low toxicity, important especially in the treatment of skin lesions and in cases where it is essential to quickly control bacteremia.
The mechanism of action of Ag + ions is not entirely known, but it is hypothesized that they exude activity as they can bind the carboxyl groups of the membrane and the thiol groups (-SH) present in enzymes and proteins, causing its denaturation. It is known that silver forms solid sulfur bonds (Ag-S) with thiol groups present in cell membrane proteins involved in energy production and ionic transport1.
It is important that no adverse effects on human cells are reported, most likely due to the presence of repair mechanisms, absent in the simplest microbial cellular organisms.
Ag+ ions do not induce resistance and are particularly active against bacterial species, also exhibiting fungi and viruses2.
Considering the remarkable antimicrobial activity of Ag +, it is essential to preserve its thermal and photochemical stability, avoiding that the species undergoes reduction processes to a metallic species with a parallel loss of efficacy.
Pavia Farmaceutici's patented technology innovation allows silver, in its active form, to stabilize, both from a thermal and a photochemical point of view.
1. Klueh, U., Wagner, V., Kelly, S., Johnson, A., Bryers, J.D. " Efficacy of Silver-Coated Fabric to Prevent Bacterial Colonization and Subsequent Device-Based Biofilm Formation." Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2000. Volume 53. p. 621-631.
2. G. Thornhill; J.B. Stahl; C.W. Opp 3M HEALTHCARE 2009; S.A. Jones, P.G. Bowler, M Walker, D. Parsons. Wound Repair and Regeneration, 12, 289, 2004.
The active agents in powder form are composed of anionic silica particles functionalized with cationic antimicrobial species adsorbed on their surface. Such species include cationic complexes of Silver and Biguanides.
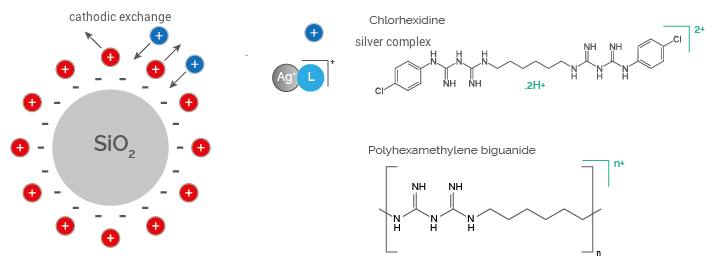
The following examples relate to some applications of silver nanoparticles employed at industrial level. Based on the characteristics of new products, colloidal silver can be replaced with new-generation photo-chemically and thermally stable silver complexes patented by Pavia Farmaceutici.
Dressings incorporating silver nanoparticles were developed in view of reducing infections. In-vitro tests showed their efficacy in eliminating Pseudomonas aeruginosa and Staphylococcus aureus colonies with >99.99% efficiency.
Patients who need artificial breathing are frequently subject to pulmonary diseases. It was observed that when treating with silver nanoparticles the internal surfaces of endotracheal tubes the formation of bacteria slows down, and the Pseudomonas aeruginosa colonization of lungs is reduced.
Studies on the antimicrobial properties of surgical masks treated with silver nanoparticles were carried out. The treated masks were able to reduce E. coli and S. aureus by nearly 100%, without irritating the skin.
Some food packaging include plastic or carton food containers. Though the FDA approved a limited number of silver-containing compounds that can come into direct contact with food, in Japan food packaging incorporating silver nanoparticles are very common.
In addition to these applications, made possible thanks to the actives designed by Pavia Farmaceutici, and to the Medical Devices, that we developed and which are already sold at drugstores, we’d like to mention the production of antimicrobial plastic materials incorporating the Silver-Sil, the production of antimicrobial acrylic varnish or polyurethane to coat plastic materials (as we did for Metalchimica) or glass containers by means of the Cosmosilver, the manufacturing of antimicrobial dressings by means of the Silver Bard and also the possibility to make antimicrobial coatings of objects and fabrics by means of the spray product Bacterial Barrier.
Furthermore, we developed (patentable) products containing high-concentration silver complexes with hydrogen peroxide or isopropyl alcohol, which could be certified as devices for surfaces and applied as disinfectants of surgery rooms and surfaces in hospitals, as well as be used on filters of any sort, including car filters.
KAdermin is a medical devices line that creates an effective protective barrier an adjuvant for the natural tissue regeneration processes and fostering the restoration of the normal physiological conditions useful to spontaneous skin regeneration.
CX complex is made from Silicon Dioxide functionalized with Silver Ions and Chlorhexidine; Thanks to its antimicrobial properties, it means that the barrier formed by the medical device is resistant to the microbial agents’ attack thanks to its antimicrobial properties, it guarantees that the barrier formed by the medical device is resistant to microbial agents’ attacks.

Indicated as an adjuvant treatment for wounds, abrasions, minor burns and especially exudative skin lesions.

Indicated for the treatment of minor wounds, cuts, minor burns and abrasions (ex. scratches).

Intimate gel with moisturising, protective action, indicated for relieving discomfort such as itching, redness and burning. Thanks to the combination of moisturising and film-forming ingredients, it helps to protect and restore physiological balance in the event of excessive development of local microbial flora and to prevent irritation.
ANTIMICROBIAL PACKAGING Reduces transmission of contact microbes


Adjuvant medical device in cream recommended for the emollient and soothing treatment of hypertrophic scars, localized systemic sclerosis, skin elasticity loss, fibrotic tissue, tissue adhesions.
Helps to restore skin elasticity

Food supplement based on avocado and soybean dry extract, vitamin E, vitamin C and PABA.
Helps to restore skin elasticity

KADERMIN VET CREAM is recommended for the treatment of minor wounds, cuts, abrasions (ex. scratches), minor burns, surgical injuries, ulcers, sores in all animals. It can be applied on all kind of damaged tissues, including mucosae.

KADERMIN VET POWDER SPRAY is a powder formulation that creates a protective barrier, on wounds, abrasions, and skin lesions especially in the presence of exuding wounds and it protects the perilesional skin from maceration phenomenons. KADERMIN POWDER SPRAY VET fosters the natural tissue repair of all animals lesions.

Kadermin Skincare powder spray is a dermoprotective, absorbent and soothing powder spray indicated to relieve skin redness and irritations such as diaper irritation , rubbing redness , red rash in skin folds and erythema.
Kadermin skincare powder spray is ideal for use especially on moist areas ( such as inguinal , axillary, interdigital, intergluteal areas, submammary fold, other skin folds).Thanks to the innovative patented ingredient Silversil, Kadermin Skincare offers an efficacious skin protection against microbial agents’s attack.

Kadermin skincare cream is a regenerative, emollient and soothing cream ideal for all the family. Its specific formulation , thanks to hydrating activity of Panthenol and Aloe, is indicated to relieve skin irritation and redness caused by shaving, hair removal, wind or sun, insect bites, cracks, rubbing and erythema of skin folds. Thanks to the innovative patented ingredient Silversil, Kadermin skincare offers an efficacious skin protection against microbial agents’s attack. Kadermin skincare cream gives your skin an immediate and pleasant sensation of freshness and skin becomes smoother and softer.

Kadermin skincare water spray thanks to the hydrating properties of Green tea and Aloe, provides a quick relief to itchy skin and it is useful in alleviating redness, irritation, dehydration of the skin and in cases of exposure to aggressive external agents (sun, wind, insect bites). Kadermin skincare water spray provides to the skin an immediate and pleasant sensation of freshness and makes it smoother and softer. It is quickly absorbed without massaging, preventing thus discomfort caused by the friction on skin. Thanks to the innovative patented ingredient Silversil offers also an efficacious skin protection against microbial agents’s attack.
Products developed by Pavia Farmaceutici are based on patented antimicrobial active compounds and can be divided into two large groups:
The table shows the products grouped by each therapeutic area.
Click please on the icon to visualize product list.
| PRODUCT | PACKAGE | DOSAGE | USE |
|---|---|---|---|
| CREAM | PET tube | 50 ml | Abrasions, macerations, traumatic wounds, decubitus ulcers, diabetic foot, athlete's foot, perilesional skin, healthy skin, burns, ulcers. |
| 125 ml | |||
| POWDER SPRAY | Aluminum bottle | 50 ml | |
| 125 ml | |||
| FOAM SPRAY | Aluminum bottle | 50 ml | |
| 125 ml | |||
| MOUSSE SPRAY | Aluminum bottle | 50 ml | |
| 125 ml |
| PRODUCT | PACKAGE | DOSAGE | USE |
|---|---|---|---|
| CREAM | PET tube | 25 ml | Vulvo-vaginitis, warts, post-partum wounds, vulvar post-surgery, vulvar care. |
| 50 ml | |||
| FOAM SPRAY | Aluminum bottle | 50 ml | |
| 125 ml | |||
| VAGINAL OVULES | Blister by 10 ovules | 10 ovules |
| PRODUCT | PACKAGE | DOSAGE | USE |
|---|---|---|---|
| MOUSSE SPRAY | Aluminum bottle | 50 ml | Red and scaly skin further to diaper use. |
| 125 ml | |||
| CREAM | PET tube | 100 ml |
| PRODUCT | PACKAGE | DOSAGE | USE |
|---|---|---|---|
| ARTIFICIAL TEARS | |||
| RINSING SOLUTION | |||
| MEDICATED EYE DROP | Corneal ulcers | ||
| HANDS-RINSING SOLUTION | |||
| PRODUCT | PACKAGE | DOSAGE | USE |
|---|---|---|---|
| GEL (WITH APPLICATOR) | PET tube | 25 ml | Anal fissures, Haemorrhoids, Ano-rectal post-surgery, care. |
| 50 ml | |||
| MINI SUPPOSITORIES | Aluminum blisters | 10 mini Suppositories | |
| MOUSSE SPRAY | Aluminum bottle | 50 ml | |
| 125 ml |
| PRODUCT | PACKAGE | DOSAGE | USE |
|---|---|---|---|
| GEL GENGIVAL | |||
| SPRAY CARE |
In addition to applications in the medical field in general, technological innovation is also applied in the production of sterile and/or antibacterial containers for pharmaceutical use. This kind of container is ideal in any field (cosmetic, medical, healthcare, pharmaceutical, veterinarian and community), where interrupting the chain of infection transmission through the handling of containers is important.
Pavia Farmaceutici's activities are carried out according to qualified protocols and standard processes to achieve the best results. We supply finished products with our own Brands and in Private Label, always developing new technologies and providing constant regulatory assistance.
The R&D Laboratory designs and develops new basic technologies that are applied to the development of new products for personal care (skincare, healthcare)
Laboratory tests allow the definition of specific products that meet customer’s requirements
Prototypes developed in the laboratory are evaluated on the basis of chemico-physical, organoleptic and microbiological characteristics that precede stability tests, that will be carried out on the industrial prototype.
The R&D laboratory is able to make formulations in gel, both on an aqueous and oily bases, emulsions, powder mixtures, mousse, foams and liquid sprays.
The information gathered during R&D represent the starting point for the industrial scale-up.
The R&D Department follows early on the production of pilot batches, supporting the toll-manufacturer of a new production step by step.
The industrial batch undergoes all the assessments necessary to define its organoleptic, chemico-physical and microbiological characteristics, its biocompatibility and stability, and finally it is clinically evaluated by the Medical Direction.
The information gathered at this stage make up the dossier required for the following certification stage, that will make the product available to patients/end users.
Products designed, developed and ready to be put on the market are certified in conformity to the rules in force, such as Directive 93/42 /EEC concerning Medical Devices Directive and the Regulation (CE) 1223/2009 on cosmetic products.
Clinical Studies
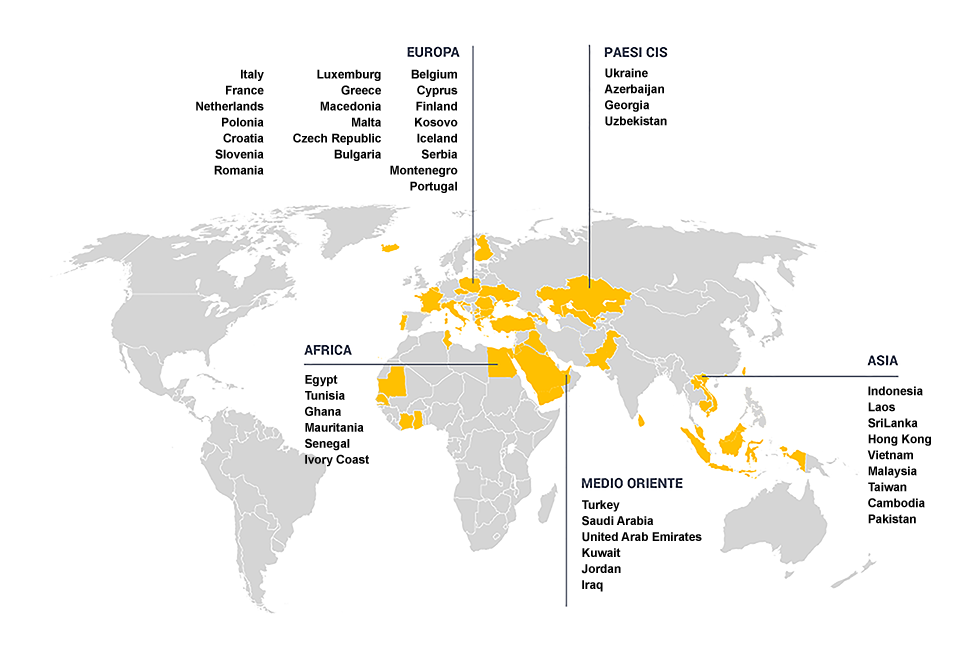
Pavia Farmaceutici decided to invest significant economic resources in an internationalization program. We are currently active in 33 countries by means of local distributors and our product are being registered in other countries.
1-5 March 2022 | Abu Dhabi
September 25-29 2016 | Florence, Italy
19 november 2019
PAVIA FARMACEUTICI s.r.l.
via Vistarino 14/F
27010 Copiano (PV) - Italy
Tel: (+39) 348 7760027
Fax: (+39) 0382 974333
Email: info@paviafarmaceutici.it
Working hours: